Link to PDF
Chemical Engineering Science 63 (2008) 5606 – 5612
Effects of magnetic field on the crystallization of CaCO3 using permanent magnets
Clifford Y. Tai ∗, Chi-Kao Wu, Meng-Chun Chang
Department of Chemical Engineering, National Taiwan University, Taipei, Taiwan
A R T I C L E I N F O A B S T R A C T
Article history:
Received 26 May 2008
Received in revised form 30 July 2008 Accepted 3 August 2008
Available online 7 August 2008
Keywords: Permanent magnet Fluidized bed Polymorphs of CaCO3 Calcite growth rate
Constant-composition technique
Permanent magnets of different intensities were used to investigate the effect of a magnetic field on the crystal growth of calcite suspended in a fluidized bed. The magnets were fixed on the suspended bed
where the crystal grew. The growth rates of calcite were measured at various levels of supersaturation (a), pH, and ionic strength (I) using the constant-composition technique. The calcite growth rates in the presence of the magnetic field were lower than those in the absence of the magnetic field, and higher
magnetic intensity yielded a lower growth rate. The effect of a magnetic field on CaCO3 polymorphism was also studied. The percentage of polymorphs was dependent on the magnetization time. Aragonite was the predominant polymorphic form in the precipitate mixture, which was induced spontaneously by changing the solution pH after the supersaturated solution had been magnetized for 48 h.
© 2008 Elsevier Ltd. All rights reserved.
Introduction
The formation of scales inside cooling water pipes and boiler walls is a common and costly problem for many industrial processes. Al- though several methods have been used for scale prevention, they usually cause some other problems, such as high cost of added chem- icals, process water being polluted by additives, waste of water by purging, and low efficiency. Among these methods, a more econom- ical and convenient method is the magnetic treatment. However, the anti-scale magnetic treatment has had a long and controver- sial history; i.e., either effective or ineffective operations have been reported (Duffy, 1977; Baker and Judd, 1996). Experiments were also performed in the laboratory to investigate the magnetic effects on CaCO3 nucleation and crystal growth via a more systematic ap- proach; however, conflicting results also exist in the literature. For example, the magnetic field would suppress (Dalas and Koutsoukos, 1989; Kobe et al., 2001), accelerate (Higashitani et al., 1993; Barrett and Parsons, 1998), or have no effect (Duffy, 1977; Hasson and Bramson, 1985) on the CaCO3 growth, thus creating a widespread controversy as to the credibility of this type of water conditioning, such as the conclusion of Herzog et al. (1989) that the effectiveness of a magnetic water treatment device was attributed to the contam- ination of solution by Fe ions, which dissolved from the device and inhibited the build-up of scale.
Although many investigators have indicated that the magnetic effect is dependent on the nature and condition of solution, such as
supersaturation, pH, and ionic strength, most of them have not con- trolled the solution properties in their studies, except for a few cases using a pH-stat technique (Dalas and Koutsoukos, 1989; Parsons et al., 1997). An additional drawback of some reported studies was measuring the deposit rate of scales on the pipe wall, which took a long time to collect any data, probably several weeks or months (Kobe et al., 2001). Recently, Tai et al. (2008) investigated the effect of a magnetic field on the calcite growth rate in a constant-composition environment. Using a commercial magnetic water treatment device (MWTD) with a magnetic intensity of 0.18 T, the crystal growth rate was measured in a fluidized bed using calcite seeds. Although the growth rate data obtained in such a manner is somewhat different from the deposit rate of CaCO3 scale grown on the pipe wall, the trend of growth phenomena should be qualitatively similar. There are two advantages of using the constant-composition technique for simulating the scale deposition, i.e., an experimental run can be completed within a few hours and the interaction between the magnetic field and one of the solution properties, including pH, supersaturation, ionic strength, ionic activity ratio, or superficial velocity can be observed by keeping other solution variables con- stant. Interesting results have been obtained using this type of study. The calcite growth rate was greatly suppressed by applying a magnetic field at low pH and supersaturation. On the other hand, the crystal growth rate seemed to increase when the solution pH was above 10.0 and the relative supersaturation was higher than
1.4. A more surprising result was that the suppression effect was
observed in a wide range of species activity ratio (R = aCa2+ /a )
3
∗ Corresponding author.
E-mail address: [email protected] (C.Y. Tai).
0009-2509/$ – see front matter © 2008 Elsevier Ltd. All rights reserved. doi:10.1016/j.ces.2008.08.004
from 0.25 to 8.5, except at the ratios near unity. Thus, the mag-
netic effect on calcite growth, either suppression or promotion, was undoubted.
In the studies of positive magnetic effect, all the authors agreed that the strength of the magnetic field should be higher than a cer- tain value to become effective. In our laboratory, the effect of a magnetic field on calcite growth was noticeable using a commer- cial MWTD with an intensity of 0.18 T (Tai et al., 2008). It was in- teresting to compare the effectiveness of lower intensity; however, the commercial MWTD, Descal-A-Matic DC-3, used in our laboratory was the model of the lowest intensity and capacity that we were able to acquire. After this, we tried to use permanent magnets to re- place the commercial device. Two magnets of different intensities, MagneGen Model 1000 and 100, were chosen in this study. At first, the operation mode was similar to that in the previous study using a commercial MWTD, i.e., the magnetic field was used to magnetize the solution. However, the calcite growth rate remained the same for a magnetization time of 3 h by using the magnet of higher inten- sity. Then, the operation mode was changed by moving the magnet to the fluidized bed, where the seed crystals suspended and grew. This meant that the magnetic field magnetized the solution and seed crystals simultaneously. This mode of operation worked well and the growth phenomena at various levels of supersaturation (a) pH, and ionic strength (I) were investigated in the presence of magnet fixed on the suspension bed.
Experimental section
-
- Apparatus and procedures for crystal growth
The experimental apparatus and procedures using a permanent magnet are similar to that reported by Tai et al. (2008) using a com- mercial MWTD. The schematic diagram of the crystallization system is shown in Fig. 1, which consists of a Plexiglas column used as the fluidized-bed crystallizer where the seed crystals grew, a storage tank where the solution composition and pH were adjusted, and a constant-composition control system which consisted of two autoti- trators for adding reactant solution and NaOH solution to keep the solution at constant composition and pH, respectively. The system was modified in two aspects, i.e., the location for the magnetic field
The experimental procedure is described briefly as follows. The typical condition for crystal growth was set as pH = 8.5, I (ionic strength)=0.018 M, a (supersaturation)=1.0, R (activity ratio)=5.54,
v (superficial velocity) = 0.047 m/s, and T (temperature) = 25 ◦C. In
the later sessions, only the discussed variable was varied over a range while the other variables kept the same. The supersaturated solution of specified conditions was prepared by mixing a certain amount of Na2CO3 and CaCl2 with deionized water. After the pH value was
adjusted using NaOH solution (Tai et al., 1999, 2008), the solution
was then pumped from the storage tank to the fluidized bed. Passing the fluidized bed, the solution would overflow back into the storage tank. When the flow rate and pH of solution became steady, calcite seed crystals of 25 g were charged into the fluidized bed and the seed crystals started to grow. Usually, it took 40–50 min for the flow rate and pH value to become steady. Once the seed crystals started to grow, a drop in Ca2+ concentration or pH value would trigger the autotitrators to replenish the reactants (0.1 M Na2CO3 and CaCl2 solutions) simultaneously, or NaOH solution into the storage tank to maintain constant composition. The titration curve, which showed the volume of CaCl2 solution added to the system, was recorded automatically and was used to estimate the linear growth rate of CaCO3. A representative titration curve obtained in the presence of a magnetic field is shown in Fig. 3, in which the titration curve was shown as almost a straight line, meaning that the crystal growth rate did not change during the growth period. As far as the impurity effect was concerned, the Fe2+, Fe3+, and Cu2+ would reduce the calcite growth rate (Takasaki et al., 1994; Herzog et al., 1989; Parsiegla and Katz, 2000). Thus, the concentrations of these ions were checked by induced coupled plasma (ICP) analysis (Optima 3100, Perkin-Elmer) after every run of the experiment to make sure that they were at an undetectable level.
-
- Determination of supersaturation and crystal growth rate
The relative supersaturation, a, defined by Nielsen and Toft (1984) was adopted as the driving force for crystal growth.
Kip 1/2
and the construction of the fluidized bed. In the original design, an
MWTD was installed in line before the fluidized bed; however, the
a = Ksp
− 1 (1)
permanent magnet was fixed on the fluidized bed. To fix the magnet, a piece of steel pipe with its inside wall coated by Teflon to prevent the dissolution of iron was inserted into the lower part of the Plex- iglas column as shown in Fig. 2. During the operation of the growth experiment, the bed of seed crystals was expanded to a height that was slightly over the top of the magnet. In the study of the column material effect, the permanent magnet was fixed outside the Plex- iglas column using adhesive tape. In both cases, the magnetic field was oriented approximately orthogonal to the direction of flow. Such
A computer program, which contains mass-action equation, mass- balance equation, and the modified Debye–Huckel equation for es- timating the activity coefficients, was used to calculate the relative supersaturation, once the total carbonate and calcium concentration and pH were known (Nancollas, 1966; Tai et al., 1993).
The crystal growth rate of calcite was estimated by the following equation developed by Tai et al. (1999) for the constant-composition operation.
an orientation was considered to be most effective for the magnetic field (Baker and Judd, 1996). Two permanent magnets of different
intensities were chosen in this study, i.e., MagneGen Model 1000 and
LM
G = 3W ([Ca
]a − [Ca2+
] ) dVa (2)
o dt
Model 100, which were manufactured by a local company in Taiwan. The intensities of the magnets were measured by a Gauss meter (Model 6010, Bell), which would record the maximum intensity dur- ing the course of measurement. The average maximum intensities of five measurements are listed in Table 1. Without fixing to any pipe, the measured nominal intensities of Model 1000 and Model 100 were 3388.0 and 1265.6 Gauss, respectively. However, the mag- netic intensity in the pipes dropped sharply, and the intensity was lower in the steel pipe. The intensity in the steel pipe was as low as 74.0 Gauss for Model 100 because the inside surface of steel pipe was coated with Teflon. It should be noted that the whole section of the steel pipe was magnetized when a magnet was fixed on it; however, the magnetic force was confined to the region covered by the magnet when a Plexiglas pipe was used.
When the titration curve, Va vs. t, is available, the crystal growth
rate can be calculated by using the slope of the curve, dVa/dt, which should be a constant. If the slope of the curve does not remain con- stant, meaning that the extent of magnetic influence keeps changing, no meaningful data can be obtained.
-
- Nucleation experiment
The nucleation experiment was designed for observing the mag- netic effects on the polymorphism of CaCO3 precipitates. A solution of low supersaturation, a = 2.23 at pH = 9.0, was prepared and cir- culated in the system without adding seed crystals. The MagneGen Model 100 magnet fixed on the Plexiglas column was used as mag- netic source to magnetize the circulating solution. After a certain
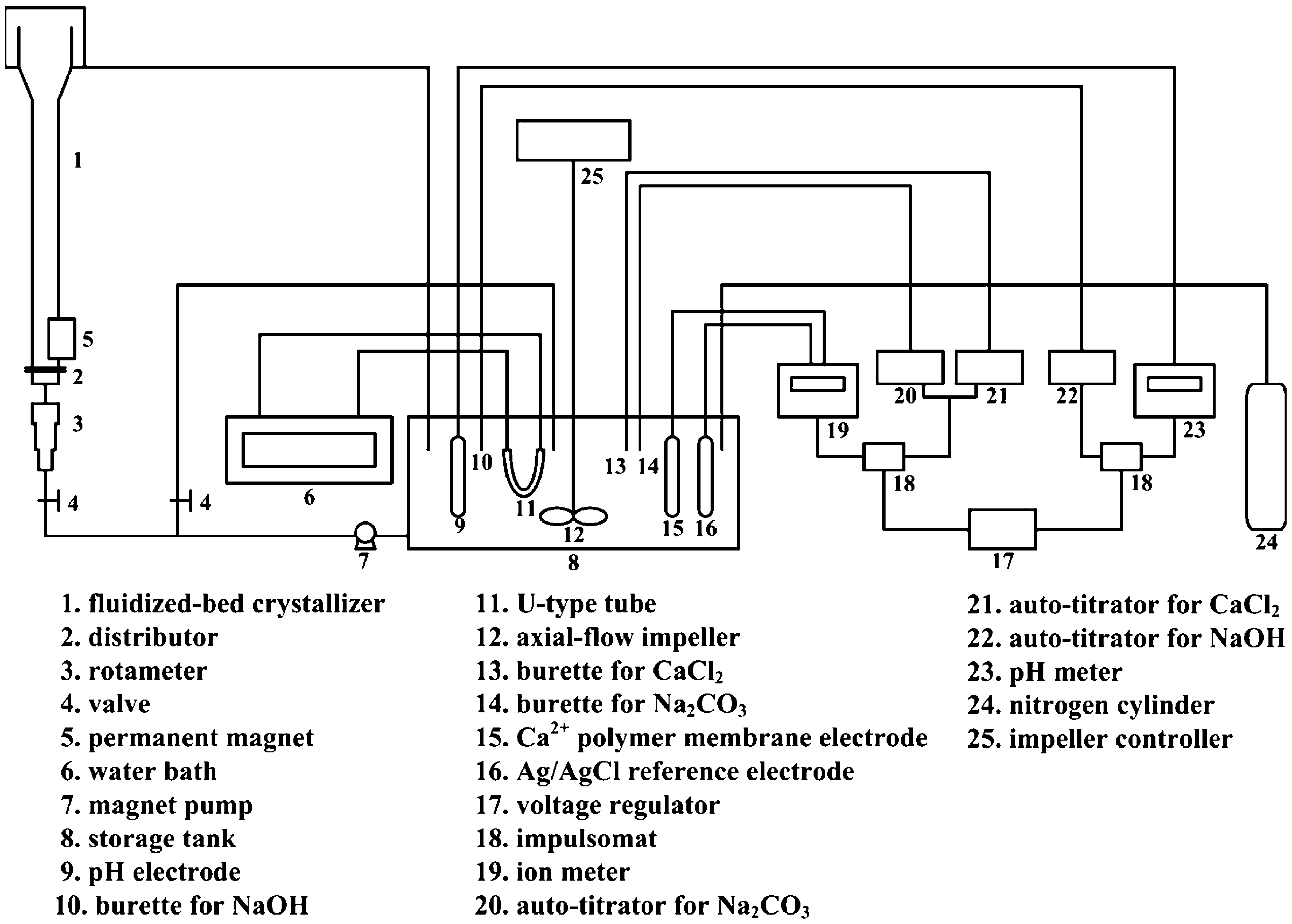
Fig. 1. Diagram of constant-composition crystallization system incorporating a permanent magnet.

Fig. 2. Specification of the fluidized-bed crystallizer composed of Plexiglas and steel pipes: (a) fluidized-bed crystallizer; (b) permanent magnet fixed on the steel pipe.
period of magnetization time, ranging from 24 to 48 h, a con- centrated NaOH solution was added to raise the pH from 9.0 to
12.5. The solubility of CaCO3 was a function of pH (Plummer and
Busenburg, 1983), and primary nucleation was induced due to the solubility change. The precipitates were filtered immediately, then washed by ethyl alcohol and deionized water and air dried. Finally,
Table 1
Intensities of magnetic field used in this experiment
| Magnet type | Magnetic field intensity (Gauss) | |||
| Nominal | Measured in Plexiglas pipe | Measured in steel pipe | ||
| Model Model | 1000100 | 3388.0 ± 305.01265.6 ± 30.0 | 1024.0 ± 41.5212.6 ± 2.9 | 356.0 ± 15.074.0 ± 3.0 |
Fig. 3. A representative titration curve for B = 356.0 Gauss, pH = 8.5, I = 0.018 M,
Fig. 4. Growth rate of calcite as a function of relative supersaturation for pH
8.5,
a = 1.2, R = 5.54, L = 774 µm, v = 0.047 m/s, T = 25 ◦ C.
I = 0.018 M,
R = 5.54,
L = 774
µm,
v = 0.047 m/s,
=
T = 25 ◦ C: (O) without magnetic
treatment, (*) magnetized for 3 h prior to growth (B = 356.0 Gauss).
the products were subjected to X-ray diffraction (XRD) analysis (Mac Science, MXP-3TXJ-7266). The percentage of polymorphs in a product mixture was determined by the direct linear analysis, as suggested by Klug and Alexander (1974). In order to collect a large enough sample for XRD analysis, several runs of the same experi- mental conditions should be performed because there was only a small amount of CaCO3 precipitate produced in each run.
Results and discussion
-
- Preliminary test on magnetic effect
In the early stage of this research, the experimental mode was similar to that using the commercial MWTD, i.e., the magnet was used to magnetize the solution (Tai et al., 2008). The results of calcite growth at various levels of supersaturation using the Model 1000 magnet to magnetize the supersaturated solution for 3 h prior to growth of seed crystals are shown in Fig. 4, in which the calcite growth rates without magnetic treatment are also presented. The calcite growth rate increased with an increase in supersaturation for either case, and there was almost no difference between these two sets of data. These results meant that the magnetic force induced by Model 1000 magnet did not work after magnetizing the solution for 3 h.
Tai et al. (2008) investigated the effect of magnetic field on the calcite growth rate using a commercial magnetic device with an intensity of 1800 Gauss, which was much higher than the highest intensity of 1024.0 Gauss used in this study. They found that the cal- cite growth rate was reduced immediately after the supersaturated solution passed through the magnetic device. As the magnetic effect did not show up in 3 h using the permanent magnet, we decided to change the operation mode. The magnet was moved to the position outside the suspended bed of seed crystals instead of the inlet pipe to
the suspended bed. In this way, the seed crystals and solution were magnetized simultaneously. Recall that 40–50 min was needed for the flow rate and pH value to become steady before the seed crystals were charged into the fluidized bed. This means that the solution was magnetized for 40–50 min prior to growth. The magnetic effect appeared immediately after the fluidized-bed crystallizer was loaded with seed crystals and the slope of titration curve stayed constant in the growth period. Experiments were then continued for studying the magnetic effects systematically using the permanent magnet.
-
- Effects of magnetic intensity at various supersaturations
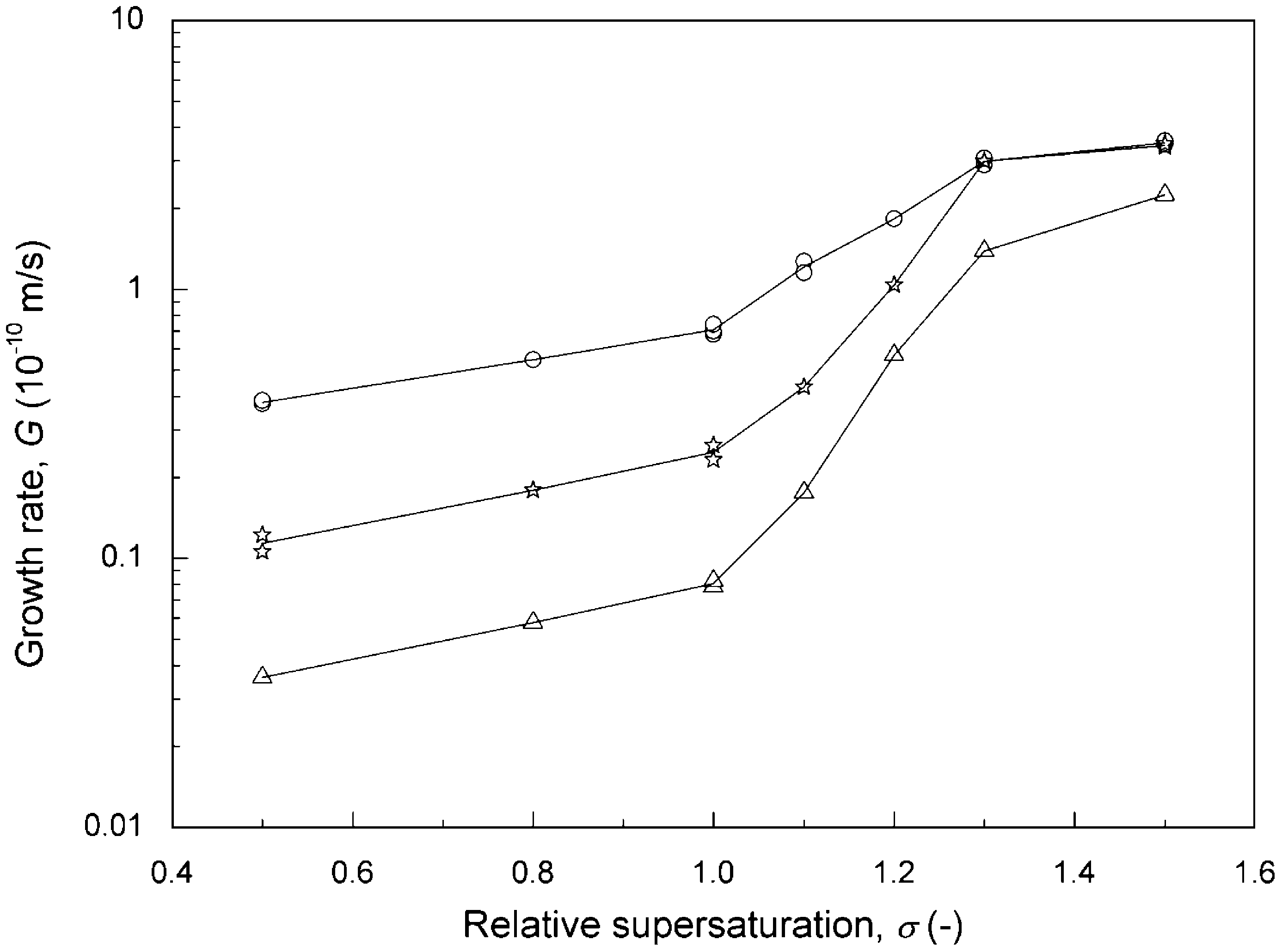
To compare the effectiveness of the magnetic field, the experi- ments were conducted at different magnetic intensities, i.e., without magnetic treatment or magnetized by Model 100 magnet and Model 1000 magnet fixed on steel pipe. The growth rates at various lev- els of supersaturation are plotted in Fig. 5. For the three cases, the calcite growth rate increased with an increase in supersaturation. The magnetic force decreased the calcite growth rate: the higher the magnetic intensity, the lower the growth rate. It is also shown in Fig. 5 that the retarded effect of a magnetic field on calcite growth was less significant in the higher supersaturation range. For instance, for the Model 100 magnet the calcite growth rate was not affected when a was higher than 1.3; for the Model 1000 magnet the growth rate was reduced by an order of magnitude at a = 0.5 and by 37% at a = 1.5. Similar results were reported by Tai et al. (2008) by using a commercial MWTD to magnetize the solution. However, they found that the calcite growth rate of treated system exceeded that of an untreated system for a higher than 1.4. The actual mechanism of the magnetic effect is not clear at the present time. We believe that the different growth behavior was due to the difference in magnetic intensity and operation mode.
Fig. 5. Growth rate of calcite as a function of relative supersaturation for pH = 8.5, I = 0.018 M, R = 5.54, L = 774 µm, v = 0.047 m/s, T = 25 ◦ C: (O) without magnetic treatment, (*) magnetized by Model 100 magnet, (L:) magnetized by Model 1000 magnet.

Fig. 6. Growth rate of calcite as a function of pH for I = 0.018 M, a = 1.0, R = 5.54, L = 774 µm, v = 0.047 m/s, T = 25 ◦ C: (O) without magnetic treatment, (L:) magnetized by Model 1000 magnet fixed on steel pipe.
-
- Effects of magnetic field at various levels of pH and ionic strength
Besides the solution supersaturation, the effect of pH and ionic strength of the supersaturated solution were also investigated. The
pH and ionic strength were adjusted by adding NaOH and NaCl, respectively. The ionic strength was calculated by I= 1 }, Ciz2, where
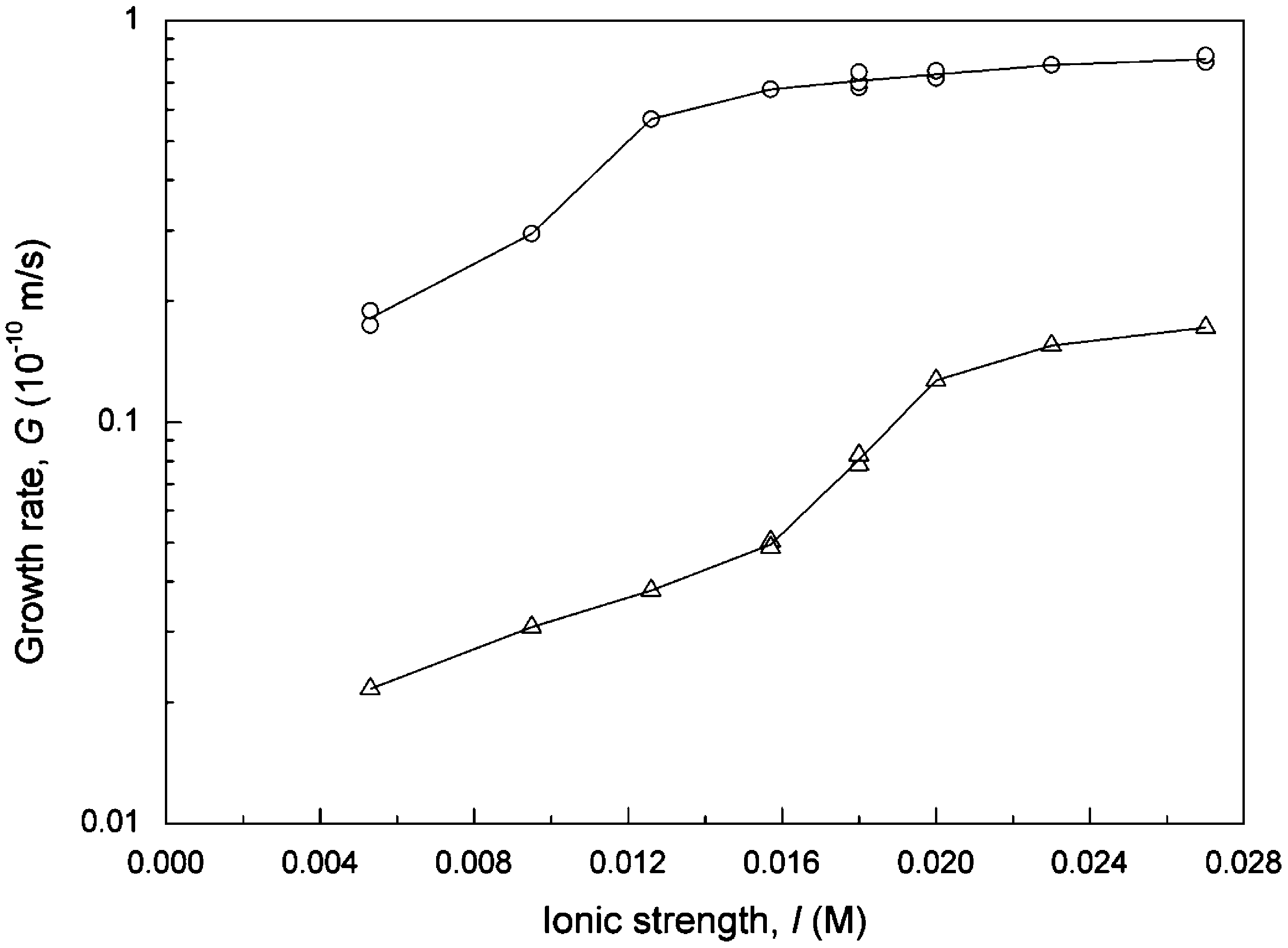
Fig. 7. Growth rate of calcite as a function of ionic strength for pH = 8.5, a = 1.0, R = 5.54, L = 774 µm, v = 0.047 m/s, T = 25 ◦ C: (O) without magnetic treatment, (L:) magnetized by Model 1000 magnet fixed on steel pipe.
growth rate was reduced by an order of magnitude at pH = 8.5 and did not increase much at higher pHs. As far as the effect of ionic strength was concerned, the growth rates increased with increasing ionic strength then tended to remain constant at higher levels for both cases. A slight difference is that the growth rate curve leveled off at a higher ionic strength for the case with magnetization.
The effects of pH and ionic strength on the calcite growth rate have been reported by Tai et al. (2008) using a commercial magnetic device to magnetize the supersaturated solution. They found that the growth rates were reduced by an order of magnitude at lower pH and increased after the pH exceeded 10.0 in the presence of magnetic field. The pH effect in this study is similar to the results in Tai et al. (2008) at lower pH, but is different at higher pH. In this experiment, the calcite growth rates of the magnetized system were still far below than that of the untreated system, as shown in Fig. 6. As far as the ionic strength is concerned, similar results were obtained for the system without magnetization in the two studies, and, as it should be, even the level-off point for the growth rate curves is about the same. However, the growth phenomena were quite different in the case of the magnetized system. The calcite growth stayed constant and was almost stopped by the magnetic force in the whole range of ionic strength as reported by Tai et al. (2008), but the crystal growth rate increased almost by an order of magnitude in the same range of ionic strength in this study ranging from 0.005 to 0.027 M, as shown in Fig. 7. Recall that the intensity of the magnetic field and operation mode were different in the two studies.
-
- Effect of bed material
Gabrielli et al. (2001) investigated the effects of a magnetic field
2 i
zi was the valence of i species and Ci included the concentration of H+, OH−, Ca2+, CaOH+, CaHCO+, HCO−, CO2−, Na+, and Cl− (Tai
on scale prevention using a home-made magnetic device fixed on
the pipe. They used different pipe materials and found that stainless
3 3 3
et al., 1993). In this experiment, NaCl was used to adjust the ionic
strength because Na+ and Cl− were already in the solution. The Model 1000 magnet was used here.
The effects of magnetic field on the calcite growth rate are pre- sented in Figs. 6 and 7, respectively, in which the growth rate ob- tained for the systems in the absence and the presence of a magnetic field were compared at different levels of pH and ionic strength, varying from 8.5 to 10.5 and from 0.005 to 0.027 M, respectively. The calcite growth rate increased with increasing pH for both cases and decreased in the presence of magnetic field. For a = 1.0, the
steel was more effective than PVC. In this experiment, growth rate
results were compared between two cases when the Model 1000 magnet was fixed on the steel and Plexiglas pipes, respectively. The results at various levels of supersaturation are presented in Fig. 8. The calcite growth rate was lower when the Plexiglas pipe was used, meaning that Plexiglas was more effective in suppressing the calcite growth. However, the difference was not really great; the greatest difference being 35% at a = 1.5. The result was not consistent with that reported by Gabrielli et al. (2001). The conflicting results were attributed to the coating of Teflon on the inside surface of steel pipe.

Fig. 8. Growth rate of calcite as a function of relative supersaturation for pH = 8.5,
I = 0.018 M, R = 5.54, L = 774 µm, v = 0.047 m/s, T = 25 ◦ C: (L:) Model 1000 magnet
fixed on steel pipe, (+) Model 1000 magnet fixed on Plexiglas pipe.

Fig. 9. Titration curve of the solution magnetized for 5 h prior to growth for B = 1024.0 Gauss, pH = 8.5, I = 0.018 M, a = 1.0, R = 5.54, L = 774 µm, v = 0.047 m/s, T = 25 ◦ C.
Referring to Table 1, the magnetic intensity measured in the steel pipe was 356.0 Gauss, which was lower than 1024.0 Gauss in the Plexiglas column. It seemed reasonable that a higher intensity field was more effective. Therefore, there is no conclusion on the effect of the bed material.
-
- Memory effect of magnetic field
In the preliminary test of magnetic field using the Model 1000 magnet, the magnetic effect was not observed in 3 h, so we continued the experiment by extending the magnetization time. The magnetic effect appeared at 4 h. When the solution was magnetized for 5 h prior to growth, the seed crystals almost did not grow at all in the first hour, as indicated by the titration curve shown in Fig. 9. Then, the growth rate of seed crystals increased gradually, as judged from the increase in the slope of titration curve. The growth rates estimated
from the titration curve for different periods of time are presented in Table 2. The growth rate increased from 0 to 0.698 × 10−10 m/s,
Table 2
Average growth rate of calcite in the solution magnetized for 5 h prior to growth
which approached to that without magnetization. It meant that the calcite growth rate was fully recovered in the period between 14 200 and 17 700 s, i.e., about 4–5 h. This growth behavior is similar to the “magnetic memory” reported by a number of investigators, which means that the antiscaling properties of the treated water will retain for some time following magnetic treatment (Baker and Judd, 1996).
-
- Effect of magnetic field on the polymorphs of CaCO3
The influence of aging time on the polymorphs of CaCO3 has been investigated in the presence of magnetic field (Kobe et al., 2002). The percentage of aragonite increased with aging time when the mag- netic field was applied. The magnetic effect which we discussed here excluded the aging effect. Recall that in this experiment the precip- itate was induced by changing pH of supersaturated solution, which had been magnetized for a period of time, and was filtered right after the nucleation occurred. The results are shown in Table 3. Runs 11 and 12 compared the percentage of polymorphs produced with- out and with magnetization, respectively, after a circulation time of 24 h. In the case without magnetization, the product was almost all calcite, which was precipitated at a pH of 12.5. This result was consis- tent with that reported by Tai and Chen (1998) who found that cal- cite was the predominant form of CaCO3 precipitated at a pH greater than 12.0 using a constant-composition method. When the solution was magnetized for 24 h before nucleation, aragonite appeared in an appreciable percentage, which was greater than 50%. If the mag- netization time was extended to 48 h, the predominant polymorphic form was aragonite and the calcite disappeared. In this study, the CaCO3 crystals were induced spontaneously, by changing pH, so the clusters existing in the supersaturated solution did not have time to change their form in the nucleation process, i.e., the clusters pos- sessed distinct form with and without magnetization, and, as a result, different polymorphs of CaCO3 crystals were produced.
-
- A possible mechanism of magnetic effect on calcite growth
Before we propose a possible mechanism of the magnetic effect on the calcite growth in this study, we would first like to summa- rize the unique features of this experiment. As far as the operation mode is concerned, the magnetic field was applied on the solution and seed crystals simultaneously, instead of the solution only. The intensities of the magnets used here were rather low, i.e., 356.0 and
74.0 Gauss, respectively, but they were effective for suppressing the calcite growth rates. The lowest intensity of the magnetic field to af- fect the calcite growth rate reported in previous literature was about 1000 Gauss when the solution was subjected to magnetization for 2h (Chibowski et al., 2003).
From the results of the nucleation experiments in this study, we postulated that different forms of CaCO3 cluster exist in the super- saturated solution after the magnetic field was applied for a period
of time. Thus, the calcite growth rate was reduced by magnetizing the supersaturated solution because the clusters unfavorable for cal- cite were not adsorbed by the crystal surface. When the solution and seed crystals were magnetized simultaneously, as in the case of
Table 3
Magnetic effect of MagneGen Model 100 on the yield of CaCO3 polymorphs
| Run no. | Magnetization by Model 100 | Circulation time (h) | Percentage of polymorphs (wt%) | ||
| Aragonite | Calcite | Vaterite | |||
| 11 | No | 24 | – | 100 | – |
| 12 | Yes | 24 | 57.8 | 42.2 | – |
| 13 | Yes | 48 | 100 | – | – |
Initial solution conditions: pH = 9.0, I = 0.018 M, a = 2.23, R = 5.54, and T = 25 ◦ C.
this study, the magnetic effect was more significant. This can be ex- plained by the theory proposed by Clontz and McCabe (1971) and Tai et al. (1992) regarding the source of secondary nuclei. It was suggested that the source of nuclei in secondary nucleation was an adsorption layer comprised solute clusters on the surface of grow- ing seed crystals. These clusters lined up in the adsorption layer awaiting incorporation into the growing crystal. It was possible that the structure of the adsorbed clusters was altered by the magnetic force just like the clusters in the solution. Then, they desorbed from the crystal/solution interface after they became the unfavorable form for calcite growth. Thus, the growth rate of the seed crystals was further reduced. However, there is no instrument available for de- tecting the structure of clusters either in the solution or adsorbed on crystal surface to make a good judgment on the magnetic effect.
Conclusion
The effect of a magnetic field on calcite crystal growth was stud- ied by fixing permanent magnets, MagneGen Model 1000 or 100, outside the fluidized bed where the seed crystals grew, using the constant-composition technique. The calcite growth rates were mea- sured at various levels of supersaturation, pH, and ionic strength. In the presence of a magnetic field, the growth rates of calcite were reduced and the suppression percentage was higher for the magnet of higher intensity. The percentage of suppression was higher at low levels of supersaturation, pH, and ionic strength, especially at low supersaturation. When the relative supersaturation, a, was 0.5, the calcite growth rate was reduced by an order of magnitude in the presence of Model 1000 magnet. In cases where the magnets were used to magnetize the solution only, the magnetic field was ineffec- tive for several hours. As far as the magnetic effect on the polymor- phic form of CaCO3 was concerned, the magnetization of solution would favor the formation of aragonite. In view of the experimental results of this growth and nucleation study, a mechanism regarding the structure alternation of CaCO3 clusters was proposed to explain the effect of magnetic field on the calcite growth rate.
Notation
ai activity of i species, kmol/m3
B intensity of magnetic field, Gauss
[Ca2+]a Ca2+ concentration of the added solution, kmol/m3 [Ca2+]o Ca2+ concentration of the original solution, kmol/m3
Va volume of the added solution, mL
W weight of seed crystals, kg
zi valence of i species, dimensionless
Greek letter
a relative supersaturation, dimensionless
Acknowledgment
The authors gratefully acknowledge financial support provided by the National Science Council of the Republic of China (Taiwan).
References
Baker, J.S., Judd, S.J., 1996. Magnetic amelioration of scale formation. Water Research 30 (2), 247–260.
Barrett, R.A., Parsons, S.A., 1998. The influence of magnetic fields on calcium
carbonate precipitation. Water Research 32 (3), 609–612.
Chibowski, E., Holysz, L., Szczes”, A., 2003. Time dependent changes in zeta potential of freshly precipitated calcium carbonate. Colloid Surface A—Physicochemical and Engineering Aspects 222, 41–54.
Clontz, N.A., McCabe, W.L., 1971. Contact nucleation of magnesium sulfate
heptahydrate. A.I.Ch.E. Symposium Series 110, 6.
Dalas, E., Koutsoukos, P.G., 1989. The effect of magnetic fields on calcium carbonate scale formation. Journal of Crystal Growth 96, 802–806.
Duffy, E.A., 1977. Investigations of water treatment devices. Ph.D. Thesis, Clemson
University.
Gabrielli, C., Jaouhari, R., Maurin, G., Keddam, M., 2001. Magnetic water treatment for scale prevention. Water Research 35 (13), 3249–3259.
Hasson, D., Bramson, D., 1985. Effectiveness of magnetic water treatment in
suppressing CaCO3 scale deposition. Industrial and Engineering Chemistry Process Design and Development 24, 588–592.
Herzog, R.E., Shi, Q., Patil, J.N., Katz, J.L., 1989. Magnetic water treatment: the effect of iron on calcium carbonate nucleation and growth. Langmuir 5, 861–867.
Higashitani, K., Kage, A., Katamura, S., 1993. Effect of magnetic field on formation
of CaCO3 particles. Journal of Colloid and Interface Science 156, 90–95.
Klug, H.P., Alexander, L.E., 1974. X-ray Diffraction Procedures. second ed. Wiley, New York.
Kobe, S., Drazic”, G., McGuiness, P.J., Strazišar, J., 2001. The influence of the magnetic
field on the crystallisation form of calcium carbonate and the testing of a magnetic water-treatment device. Journal of Magnetism and Magnetic Materials 236, 71–76.
Kobe, S., Drazic”, G., Cefalas, A.C., Sarantopoulou, E., Strazišar, J., 2002. Nucleation
and crystallization of CaCO3 in applied magnetic fields. Crystal Engineering 5, 243–253.
Nancollas, G.H., 1966. Interactions in Electrolyte Solutions. Elsevier, Amsterdam. Nielsen, A.E., Toft, J.M., 1984. Electrolyte crystal growth kinetics. Journal of Crystal
Growth 67, 278–288.
Parsiegla, K.I., Katz, J.L., 2000. Calcite growth inhibition by copper(II). II. Effect of solution composition. Journal of Crystal Growth 213, 368–380.
Parsons, S.A., Wang, B.L., Judd, S.J., Stephenson, T., 1997. Magnetic treatment of
calcium carbonate scale—effect of pH control. Water Research 31, 339–342.
Plummer, L.N., Busenburg, E., 1983. The solubilities of calcite, aragonite, and vaterite in CO –H O solutions between 0 and 90 ◦C, and an evaluation of the aqueous
2 2
Ci concentration of i species, kmol/m3
G linear growth rate, m/s
I ionic strength, kmol/m3
Kip ionic product
Ksp solubility product
- mean crystal size, m
- molecular weight, kg/kmol
R Ca2+ to CO2− activity ratio, dimensionless
t time, s
T temperature, ◦C
v superficial velocity, m/s
model for the system CaCO3 –CO2 –H2 O. Geochimica et Cosmochimica Acta 46, 1011–1040.
Tai, C.Y., Chen, F.B., 1998. Polymorphism of CaCO3 precipitated in a constant- composition environment. A.I.Ch.E. Journal 144 (8), 1790–1798.
Tai, C.Y., Wu, J.F., Rousseau, R.W., 1992. Interfacial supersaturation, secondary, nucleation and crystal growth. Journal of Crystal Growth 116, 294–306.
Tai, C.Y., Chen, P.C., Shih, S.M., 1993. Size-dependent growth and contact nucleation
of calcite crystals. A.I.Ch.E. Journal 39, 1472.
Tai, C.Y., Chien, W.C., Chen, C.Y., 1999. Crystal growth kinetics of calcite in a dense fluidized-bed crystallizer. A.I.Ch.E. Journal 145 (8), 1605–1614.
Tai, C.Y., Chang, M.C., Shieh, R.J., Chen, T.G., 2008. Magnetic effects on CaCO3 crystallization using a commercial magnetic treatment device. Journal of Crystal
Growth 310, 3690–3697.
Takasaki, S., Parsiegla, K.I., Katz, J.L., 1994. Calcite growth and the inhibiting effect of iron(III). Journal of Crystal Growth 143, 261–268.